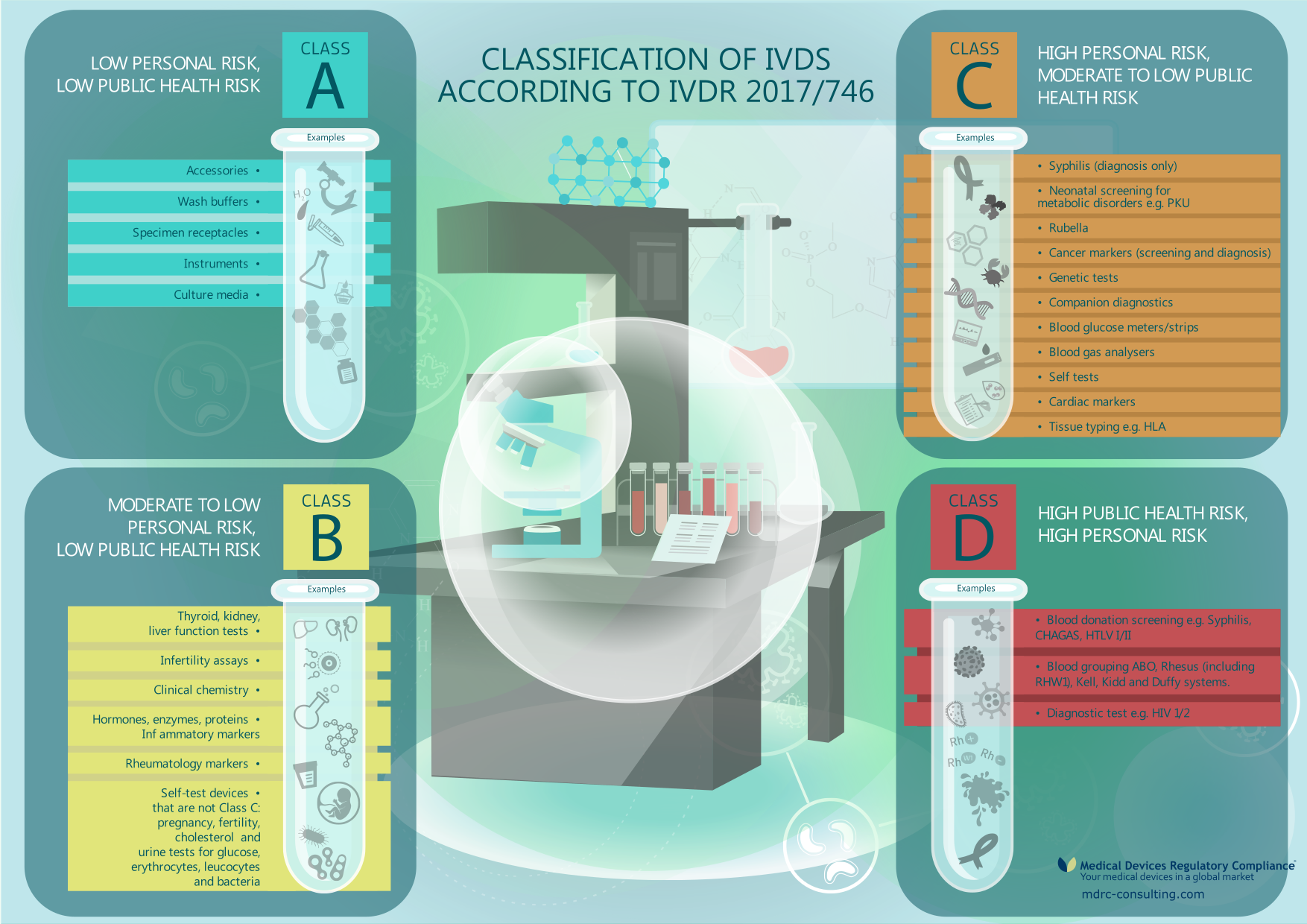
Vitro Diagnostic Medical Devices Regulation . This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/745 on medical devices. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/746 on in vitro diagnostic medical.
from mdrc-consulting.com
Regulation (eu) 2017/746 on in vitro diagnostic medical. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 on medical devices. The in vitro diagnostic devices regulation applies since 26 may 2022.
Europe's IVD regulatory approval process MDRC
Vitro Diagnostic Medical Devices Regulation This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/745 on medical devices. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/746 on in vitro diagnostic medical. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal.
From www.researchgate.net
(PDF) The invitro diagnostics regulation (IVDR) From oversight to Vitro Diagnostic Medical Devices Regulation The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/745 on medical devices. Regulation (eu) 2017/746 on in vitro diagnostic medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing.. Vitro Diagnostic Medical Devices Regulation.
From dicentra.com
EU In Vitro Diagnostic Medical Device Regulation dicentra Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/745 on medical devices. It repeals directive 98/79/ec of the european parliament and of the council. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. The in vitro diagnostic devices regulation applies since 26 may 2022. This guideline describes the information that should be presented in the quality part. Vitro Diagnostic Medical Devices Regulation.
From www.jamasoftware.com
Guide to New EU In Vitro Diagnostic Regulations Jama Software Vitro Diagnostic Medical Devices Regulation The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/745 on medical devices. Regulation (eu) 2017/746 on in vitro diagnostic medical. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april. Vitro Diagnostic Medical Devices Regulation.
From www.bsigroup.com
In Vitro Diagnostic Regulation IVDR Medical Devices BSI America Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/745 on medical devices. The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/746 on in vitro diagnostic medical. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework. Vitro Diagnostic Medical Devices Regulation.
From operonstrategist.com
Guide to In Vitro Diagnostic Medical Device Regulation (IVDR) IVD Vitro Diagnostic Medical Devices Regulation This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/745 on medical devices. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The in vitro diagnostic devices regulation applies since 26 may. Vitro Diagnostic Medical Devices Regulation.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Vitro Diagnostic Medical Devices Regulation Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. Regulation (eu) 2017/745 on medical devices. It repeals directive 98/79/ec of the european parliament and of the council. The in vitro diagnostic devices regulation applies since 26 may 2022. This guideline describes the information that should be presented in the quality part. Vitro Diagnostic Medical Devices Regulation.
From www.mdpi.com
Diagnostics Free FullText A Systematic Database Approach to Vitro Diagnostic Medical Devices Regulation The in vitro diagnostic devices regulation applies since 26 may 2022. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 on in vitro diagnostic medical. Regulation (eu) 2017/745 on medical devices. It repeals directive 98/79/ec of the european parliament and of the council. Council directive. Vitro Diagnostic Medical Devices Regulation.
From www.unitedlanguagegroup.com
The EU’s In Vitro Diagnostic Regulation Explained Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 on in vitro diagnostic medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. It repeals directive 98/79/ec of the european parliament and of the. Vitro Diagnostic Medical Devices Regulation.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 on in vitro diagnostic medical. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. This guideline describes the information that should be presented in the. Vitro Diagnostic Medical Devices Regulation.
From www.thermofisher.com
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/745 on medical devices. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for. Vitro Diagnostic Medical Devices Regulation.
From www.tuvsud.com
EU In Vitro Diagnostic Medical Device Regulation TÜV SÜD Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. It repeals directive 98/79/ec of the european parliament and of the council. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 on. Vitro Diagnostic Medical Devices Regulation.
From www.nsf.org
In Vitro Diagnostic Medical Device Regulation… NSF International Vitro Diagnostic Medical Devices Regulation The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/746 on in vitro diagnostic medical. It repeals directive 98/79/ec of the european parliament and of the council. Regulation (eu) 2017/745 on medical devices. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing.. Vitro Diagnostic Medical Devices Regulation.
From bzt-ar.com
IVDR BZTAR Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for. Vitro Diagnostic Medical Devices Regulation.
From management-forum.co.uk
Introduction to the InVitro Diagnostic Regulation (IVDR) Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 on medical devices. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 on in vitro diagnostic medical. The in. Vitro Diagnostic Medical Devices Regulation.
From www.slideshare.net
Regulation of In Vitro Diagnostic Medical Devices Transition to the… Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. It repeals directive 98/79/ec of the european parliament and of the council. The in vitro diagnostic devices regulation applies since 26 may 2022. Regulation (eu) 2017/746 on in vitro diagnostic medical. This guideline describes the information that. Vitro Diagnostic Medical Devices Regulation.
From www.eclevarmedtech.com
Transitioning from IVDD to IVDR In Vitro Diagnostic Regulation Vitro Diagnostic Medical Devices Regulation Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. Regulation (eu) 2017/745 on medical devices. This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 on in vitro diagnostic medical. Regulation (eu) 2017/746 of the european parliament. Vitro Diagnostic Medical Devices Regulation.
From www.complianceandrisks.com
EU MDR & IVDR Regulation Complete Checklist To Be Ready Vitro Diagnostic Medical Devices Regulation Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 on medical devices. Council directive 90/385/eec (3) and council directive 93/42/eec (4) constitute the union regulatory framework for medical devices, other. This guideline describes the information that should be presented in the quality part. Vitro Diagnostic Medical Devices Regulation.
From www.sirris.be
New IVDR regulation on invitro diagnostic medical devices explained Vitro Diagnostic Medical Devices Regulation This guideline describes the information that should be presented in the quality part of a marketing authorisation dossier for a medicinal. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 on medical devices. Regulation (eu) 2017/746 on in vitro diagnostic medical. The in. Vitro Diagnostic Medical Devices Regulation.